Extinguish at 121 degrees Celsius for 10-15 minutes Bacterial manipulation is the most commonly used method of eradication Bacterial methods and standard conditions. However, why choose 121 degrees Celsius instead of 120 degrees Celsius or 122 degrees Celsius?
1、 History and standard traceability
The early adoption of the Fahrenheit temperature scale in the United States will be discontinued The bacterial temperature is set to 250 ° F, which is converted to 121 ° C in Celsius. This standard is gradually gaining popularity in the country Internationally recognized and widely applied.
、 ine Accurately kill fine Bacillus spores
Fine Bacterial spores, as the most difficult to clear and remove dormant microorganisms, c
an achieve high temperatures at 121 ℃ The balance point of effective killing. At 120 ° C, the time required to kill spores is long, which not only reduces efficiency but also poses a risk of missed killing; Although 122 ° C can shorten the extinction to a certain extent Bacterial time, but the improvement in efficiency and rate is limited.
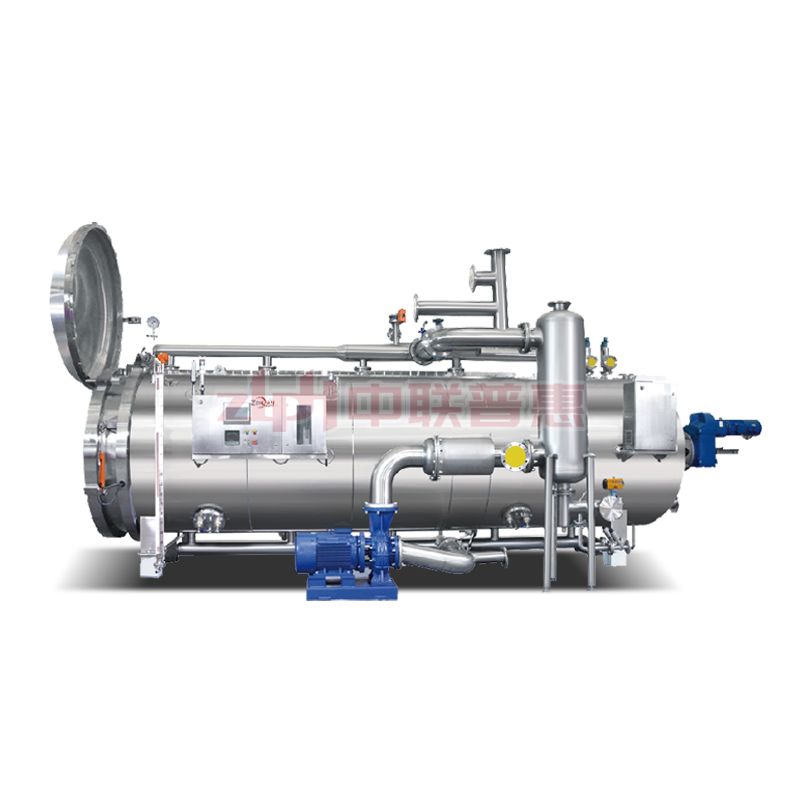
3、 Adapt to extinction Bacterial equipment and technology
fixed
Extinguish in saturated steam During the bacterial process, 121 ℃ corresponds to a gauge pressure of 0.1MPA, which is a normal pressure level The mushroom pot is easy to achieve and safe Full pressure setting
In the fields of food safety and pharmaceutical manufacturing, commercial sterilization is a critical process that ensures products are completely free of viable microorganisms, extending shelf life and guaranteeing consumer safety. At the heart of this process lies a seemingly simple number: 121°C (approximately 250°F). Why has this specific temperature become the global gold standard in the sterilizer industry?
Microbial Heat Resistance and Thermal Death Time
The primary goal of commercial sterilization is to destroy all microorganisms, including bacterial spores, which are the most heat-resistant forms of microbes. Scientific research has established that 121°C under specific pressure conditions (typically 15 psi or 1.03 bar) is effective in killing even the most heat-resistant bacterial spores—those of Clostridium botulinum.
The toxin produced by this microorganism is one of the most lethal natural toxins known, potentially fatal even in minute quantities. Studies show that at 121°C, the thermal death time (D-value) for C. botulinum spores is approximately 0.1-0.2 minutes, meaning 90% of the spores die in about 12 seconds at this temperature. Applying the 12D concept (reducing spore population by 10^12 times) requires about 2.4 minutes of exposure time. This is why many commercial sterilization processes maintain 121°C for a minimum of 3 minutes.
The Synergistic Effect of Temperature and Pressure
121°C does not exist in isolation; it is intrinsically linked to pressure. At standard atmospheric pressure, water boils at 100°C. However, by increasing pressure—as inside a retort sterilizer—the boiling point of water rises accordingly. This pressure-temperature relationship follows the physical laws of steam tables. 121°C corresponds to the saturated steam pressure of approximately 15 psi. This combination creates an ideal environment for heat transfer, ensuring heat penetrates the product and its packaging uniformly and efficiently.
The Evolution of Commercial Sterilization Equipment: From Basic to Precision
The Advancement of Retort Technology
The retort sterilizer, or autoclave, is the core equipment for executing commercial sterilization. Modern retort sterilizers have evolved into highly sophisticated systems offering precise temperature control and uniform heat distribution. These devices come in several designs:
1、Static Retorts: Traditional designs suitable for canned or pouched products.
2、Rotating/Agitating Retorts: Enhance heat transfer through mechanical movement, reducing process time.
3、Water Immersion Retort Systems: Particularly suited for flexible packaging.
4、Steam-Air Mix Systems: Provide more uniform temperature distribution, especially for complex packaging.
Each retort sterilizer design shares a common goal: to ensure all areas of the product reach and maintain the core temperature of 121°C for a predetermined time (typically at least 3 minutes, often longer depending on product and pack size).
Intelligent Control in Modern Sterilization Systems
Contemporary commercial sterilization facilities employ advanced monitoring and control systems, including:
Multi-point temperature monitoring to ensure thermal uniformity throughout the entire load.
F0-value calculation, quantifying the lethality of the sterilization process.
Automatic pressure compensation to prevent package distortion or damage.
Comprehensive data logging and traceability systems to meet stringent regulatory requirements.
These technological advancements guarantee the precise application of 121°C, regardless of product type or packaging format.
Special Challenges and Solutions in Bird's Nest Sterilization
The Unique Properties of Bird's Nest
Bird's Nest, as a high-value natural product, presents specific challenges for its sterilization:
Nutrient Sensitivity: Rich in proteins and bioactive compounds prone to thermal degradation.
Structural Integrity: The unique fibrous structure must be protected from thermal damage.
Sensory Characteristics: Color, texture, and delicate flavor must be preserved as much as possible.
Optimized Sterilization Methods for Bird's Nest
For Bird's Nest Sterilization, the industry employs specialized protocols:
1、Precise Temperature Control: Strictly controlling the exposure time at 121°C to balance safety and quality preservation.
2、Pretreatment Techniques: Such as pre-soaking and pH adjustment, to enhance protection of heat-sensitive components.
3、Customized Temperature Profiles: Often employing a staged heating approach to minimize thermal shock to the delicate nest structure.
4、Advanced Packaging Solutions: Using materials that can withstand retort sterilizer conditions while protecting the product's unique qualities.
The Art of Balancing Temperature and Time
The success of Bird's Nest Sterilization hinges on finding the precise application of 121°C—long enough to ensure commercial sterilization, yet short enough to preserve the product's intrinsic value. This requires a deep understanding of D-value and Z-value concepts, where the Z-value represents the temperature change required to change the D-value by a factor of 10 (typically around 10°C for many spores).
For bird's nest, operators might use a slightly higher temperature (e.g., 125°C) for a shorter time, or a slightly lower temperature for a longer duration, depending on product form and packaging. However, 121°C remains the foundational reference point and starting point for process development.
Global Standards and the Regulatory Framework for Commercial Sterilization
International Standards and Guidelines
Commercial sterilization processes are strictly regulated under major global standards, including:
The U.S. Food and Drug Administration (FDA) regulations for Low-Acid Canned Foods (LACF).
The European Union's Regulation (EC) No 852/2004 on the hygiene of foodstuffs.
Standards set by the Codex Alimentarius Commission.
Country-specific food safety regulations and pharmacopeias (e.g., USP for pharmaceuticals).
All these standards recognize 121°C as a critical parameter for ensuring commercial sterility, particularly for low-acid foods with a pH above 4.6.
Validation and Documentation Requirements
Implementing a commercial sterilization process requires comprehensive validation:
Heat Distribution Studies: Ensuring temperature uniformity throughout the retort sterilizer chamber.
Heat Penetration Tests: Verifying that the "coldest point" within the product achieves the target 121°C for the required time.
Microbiological Challenge Studies: Confirming process effectiveness using biological indicators (e.g., spores of Geobacillus stearothermophilus).
Ongoing Monitoring: Maintaining process control via calibrated temperature sensors and data acquisition systems.
Beyond Tradition: Alternative and Complementary Technologies
While steam sterilization at 121°C is the gold standard, other technologies serve as complements or alternatives in specific applications:
High-Pressure Processing (HPP)
Uses high isostatic pressure rather than heat to inactivate microbes.
Suitable for heat-sensitive products.
Cannot achieve "commercial sterility" for shelf-stable products; requires refrigeration.
Uses ionizing radiation to destroy microorganisms.
Applicable for certain product categories like spices.
Faces challenges with consumer acceptance and regulatory limitations in some regions.
Pulsed Electric Field (PEF)
An emerging technology using short bursts of high voltage.
Primarily for liquid products.
Still under commercial validation for broad application.
However, for products requiring true ambient-temperature stability and commercial sterility—such as canned goods, certain pharmaceuticals, and medical devices—steam sterilization at 121°C remains irreplaceable.
Industrial Applications: Sterilization Parameters for Diverse Products
Food Industry Applications
Low-Acid Canned Foods: 121°C for 3-5 minutes or longer (dependent on can size and product characteristics like viscosity).
Liquid Products (e.g., soups, broths): May require shorter processes due to better convection heating.
Viscous or Solid-Pack Products: Require longer processes to ensure heat penetrates to the geometric center.
Pharmaceutical and Medical Industry
Aqueous Injectables: Often 121°C for 15 minutes or more.
Medical Devices: Cycle times vary greatly based on device composition, density, and packaging.
Biological Waste: 121°C for 30-60 minutes to ensure complete inactivation of all biological materials.
Specialized Products like Edible Bird's Nest
Canned Bird's Nest: Precisely controlled time at 121°C is critical to balance safety with preserving delicate texture and nutrients.
Ready-to-Drink Bird's Nest Beverages: Parameters are adjusted based on pH, packaging form (bottle vs. can), and desired shelf-life.
Optimization and Innovation in Sterilization Processes
Energy Efficiency and Environmental Considerations
Modern retort sterilizer designs prioritize sustainability:
Heat Recovery Systems: Capturing waste heat to pre-heat water for subsequent cycles.
Advanced Insulation: Significantly reducing thermal energy loss.
Water Management: Implementing water recirculation and reuse systems to minimize consumption.
Process Automation and Industry 4.0 Integration
Smart technologies are transforming commercial sterilization:
Predictive Maintenance: Using sensor data to anticipate equipment needs, reducing downtime.
Adaptive Process Control: Real-time adjustment of temperature and pressure based on in-process data.
Blockchain for Traceability: Creating immutable records from farm to fork, enhancing supply chain transparency and safety.
Customization for Premium Products
For high-value products like edible Bird's Nest, sterilization is increasingly bespoke:
Product-specific temperature-time profiles based on unique microbial load and thermal properties.
Load pattern optimization for specific package sizes and shapes to maximize efficiency.
Integration of non-destructive testing (e.g., vision systems) to ensure final product quality post-sterilization.
The Enduring Significance and Future Outlook of 121°C
121°C stands as the benchmark temperature for commercial sterilization, its efficacy proven over more than a century of application. From basic retort sterilizers to highly advanced sterilization systems, this parameter remains central to ensuring product safety. For premium applications like Bird's Nest Sterilization, precisely controlling exposure time at 121°C is the key to balancing microbiological safety with product quality preservation.
As technology advances, we can anticipate even more precise temperature control, smarter process optimization through AI and machine learning, and the continued development of novel sterilization methods. Nevertheless, steam sterilization at 121°C is likely to remain the gold standard for commercial sterilization for decades to come, especially for products requiring long-term, ambient-temperature stability.
For industry professionals, understanding the science behind 121°C, mastering the operation of modern retort sterilizers, and developing the ability to customize sterilization protocols for specific products like bird's nest remain fundamental requirements for ensuring product safety, quality, and commercial success.
The field of commercial sterilization continues to evolve, but the simple yet powerful parameter of 121°C will undoubtedly continue to play a central role in global food safety and product protection. Whether for large-scale food producers, pharmaceutical companies, or specialized Bird's Nest processors, a deep understanding and precise execution of this process are keys to achieving excellence.